Model Of An Atom

Bohr Model Of The Atom

Atomic Structure The Changing Models Of Atom

Quantum Mechanical Model Of The Atom Part 01 Youtube

Rutherford Atomic Model Observations And Limitations In Detail
:max_bytes(150000):strip_icc()/model-of-an-helium-atom-107885332-5a522798b39d03003758e108.jpg)
How To Make A Model Of An Atom

Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com
Sufficient electrons surround the nucleus.
Model of an atom. The Plum Pudding Model, which was devised by J.J. Developing models of atoms Dalton’s model (1803) John Dalton thought that all matter was made of tiny particles. While preparing the element atom model, students can learn about the element, protons, neutrons, electrons and energy levels.
The most striking property of the atom was its permanence. It needed slight modifications. 3D atom models are a common science project and craft made to help understand how certain atoms work.
One of the most important contributions to atomic theory (the field of science that looks at atoms) was the development of quantum theory. Models of the Atom. Rutherford Model of an atom (1) Nucleus is very small in size, carries positive charge and in which the entire mass of the atom is concentrated.
Using these models, they investigate the makeup of atoms, including their relative size. The quantum mechanical model is based on mathematics. Although it is more difficult to understand than the Bohr model, it can be used to explain observations made on complex atoms.
The first attempt to construct a physical model of an atom was made by William Thomson (later elevated to Lord Kelvin) in 1867. The diameter of a hydrogen atom is roughly 100,000 times larger than a proton. Bohr model of the atom was proposed by Neil Bohr in 1915.
The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. The Principal Quantum Number. There were a lot of problems related to the calculation of the mass of the nucleus, although Rutherford’s model could explain the scattering of alpha.
Atom models aren't too hard to build and this article shares a few different atoms that you can create. •decided to make a new model based off of Rutherford's model, but changed the orbit of the electron •created energy levels in the atom, where only a certain amount of electrons could fit on one energy level of the atom •used Planck's ideas in order to create quantum mechanics. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.
Explain what holds an atom together. To explain the two types of static electricity, he suggested that the. This force binds the electrons inside a static while surrounding the nucleus, meaning that an external.
Rutherford's model of an Atom was undoubtedly a breakthrough in Atomic studies. Rutherford model, also called Rutherford atomic model, nuclear atom, or planetary model of the atom, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford. There has been a variety of atomic models throughout history of atomic physics, that refers mainly to a period from the beginning of 19th century to the first half of th century, when a final model of atom which is being used nowadays (or accepted as the most accurate one) was invented.
Make sure to subscribe to my channel. Model of the Atom An atom is a building block of matter that cannot be broken apart using any chemical means. Called atoms, which he imagined as tiny solid balls.
And it is a suitable project for students learning the basics of chemistry. Despite all this, Bohr’s is probably still the model of the atom you’re most familiar with, since it’s often the one first introduced during high school or secondary school chemistry courses. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
Thomson’s so-called “plum pudding model” of the atom was incorrect. Students are then asked to form molecules out of atoms, much in the same way they constructed atoms out of the particles that atoms are made of. (2) Since electrons have negligible mass ,the mass of the atom is mainly due to protons and neutrons.
The first quantum number describes the electron shell, or energy level, of an atom. This model was known as the 'plum pudding' model. Also, use our chapter notes to study Rutherford’s alpha-particle scattering experiment and more.
We know a structure of an atom consists of electrons, protons, and neutrons. A model of an atom is not as small as an actual atom. It came into existence with the modification of Rutherford’s model of an atom.
The Bohr model and all of its successors describe the properties of atomic electrons in terms of a set of allowed (possible) values. Here's a closer look at the Bohr Model, which is sometimes called the Rutherford-Bohr Model. The first model of the atom was developed by JJ Thomson in 1904, who thought that atoms were composed purely of negatively charged electrons.
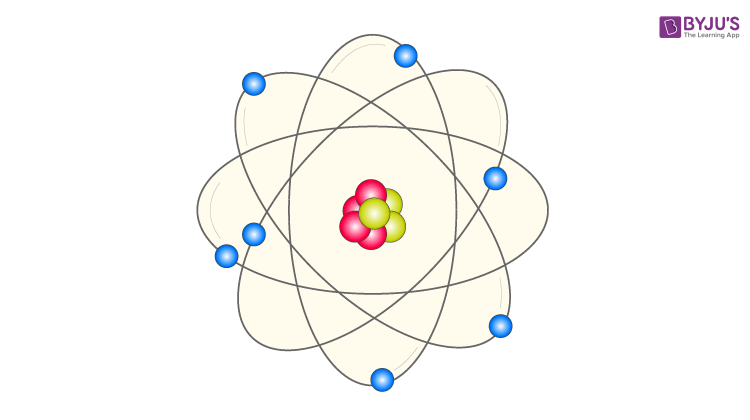
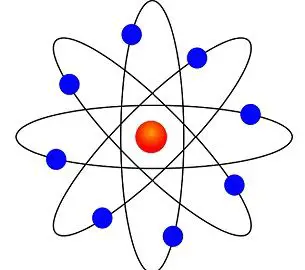
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity. Model of an atom. According to Thomson Model of an atom:.
Shop Skateboards, Wheels, Trucks & More!. Skip to main content. The Chadwick model of an atom.
He called this region of the atom as a nucleus. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. The Bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases.
This web page shows the scale of a hydrogen atom. Chadwick in this way prepared the way. The Rutherford model is a model of the atom named after Ernest Rutherford.
The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. We also know that atomic weight is a product of. The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models.
With our sample questions and solutions, learn topics like Dalton’s atomic theory, J. 2)The positive and negative charges in an atom are equal in magnitude,due to. The Bohr Model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons.
An atom is the smallest particle of any element that still retains the characteristics of that element. But I'm probally wrong. Describe the basic structure of an atom.
Nuclear reactions can alter atoms. In the Bohr model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged).
Rutherford directed the famous Geiger-Marsden experiment in 1909, which suggested, according to Rutherford’s 1911 analysis, that J. Two of the electrons are in the first energy level while the other five are in the second energy level. The plum pudding model After discovering the electron in 17, J J Thomson proposed that the atom looked like a plum pudding.
How is a model of an atom different from a real atom?. Thompson’s cathode ray experiment etc. Electron spin and the Stern-Gerlach experiment.
Two models of atomic structure are in use today:. The Bohr model of the atom was the first complete physical model of the atom. It was difficult to imagine any small solid entity that could not be broken.
Quantum numbers These four quantum numbers are used to describe the probable location of an electron in an atom. Apr 8, 16 - Explore The Homeschool Scientist's board "Atom Models", followed by 147 people on Pinterest. It’s quite handy for explaining chemical bonding and the reactivity of some groups of elements at a simple level.
A model of an atom is more complex than a real atom. Thompson by the end of the 19th century, was a crucial step in the development of atomic physics. This atomic model was discovered through the bombardment experiment of alpha particles on gold foil.
The central region of an atom has a very small positively-charged nucleus which contains almost all the mass of the atom. The following article will explain the timeline of the changing models of atom and the current model of the atomic structure. The electron cloud model is currently the most sophisticated and widely accepted model of the atom.
He defined an atom as the smallest indivisible particle. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Rutherford’s model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.
Neutrons were involved in creating nuclear explosions and nuclear energy, for it is by bombardment with high-energy neutrons that scientists first learned how to split an atom. Atomic Model Construction. Michael Fowler, University of Virginia.
A 3D atom model can be useful to demonstrate in a classroom or use to explain when giving a lesson about atoms. Our Vans Skate Pro Shop’s are located exclusively in Vans Retail stores in over 190 locations nationwide. Atomic models edit | edit source.
Death-rays, free electron lasers, proton cancer therapy.It’s a time-traveling experience not unlike what one might experience riding near the speed of light on one of Grimm’s charged particles, through a subatomic world where time has warped and anything sounds. After reading this section you will be able to do the following:. James Chadwick and Discovery of Neutrons.
A neutron is a positively charged particle with an atomic mass of 1that resides in the nucleus of an atom. A model of an atom is the same as a real atom. The atom model can be used to teach students about the structure of elements;.
According to the Rutherford atomic model:. The quantum mechanical model of the atom Introduction to the quantum mechanical model of the atom:. A model of an atom is not as big as an actual atom.
Overview of the Bohr Model Niels Bohrproposed the Bohr Model of the Atom in 1915. 1)An atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thinking about electrons as probabilistic matter waves using the de Broglie wavelength, the Schrödinger equation, and the Heisenberg uncertainty principle.
Students also practice adding and subtracting electrons from an atom and determining the overall. In the ICSE Class 8 Chemistry Chapter 4 Atomic Structure, you will learn about the model of an atom. Choose an atom with an atomic number of at least 11, since it has at least three energy level rings source:.
Though some of his conclusions were incorrect, his contributions were vital. However, atoms consist of even smaller particles. The classic model of an atom was given by Ernest Rutherford called the Rutherford atomic model or Rutherford model of the atom.
This was accurately presented after several scientists came up with different models. See more ideas about Atom model, Atom, Atom project. The Refined Bohr Model.
Try Prime EN Hello, Sign in Account & Lists Sign in Account & Lists Orders Try Prime Cart. This nucleus is tiny and the rest of the atom is mostly empty space. These theories were later validated by observations made with the electron microscope.
OK let's make that model. The model that describes the movement of water through the reservoirs of the Earth System is called the _____ cycle. The Bohr model and the quantum mechanical model.
Hope you guys like my video. Therefore, if we make a proton the size of the picture above, 1000 pixels across, then the electron orbiting this proton is located 50,000,000 pixels to the right (but could be found anywhere in the sphere around the proton at that distance). You can find the table in an encyclopedia, a science textbook or online.
It described the overall structure of the atom, how atoms bond to each other, and predicted the spectral lines of hydrogen. (Its the Speaker Knockerz) Today's Model + View Thank You For Your Time!. It retains the concept of the nucleus from Bohr and Rutherford's models, but introduces a different definition of the motion of electrons around the nucleus.
Before you begin, look through the Periodic Table of Elements and pick an atom. Students use gumdrops and toothpicks to make lithium atom models. Neils Bohr’s model a nitrogen atom.
What is an atom composed of?. However, it wasn't completely correct. It still has its uses too;.
The students have started new specifications of OCR AS Chemistry A (H034),AQA AS Chemistry (7404), Edexcel AS Chemistry (8CH0) and CIE AS/A-level Chemistry (9701).All specifications require fundamental understanding of the changing models of atom and atomic structure. Dalton's atomic model sets up the building blocks for others to improve on. A casual chat with Terry Grimm, of the Lansing-based, high-tech company, Niowave, can induce a state of technological whiplash.
Other models of the atom (ESAAT) Although the most commonly used model of the atom is the Bohr model, scientists are still developing new and improved theories on what the atom looks like. A Bohr model of a nitrogen atom could look like this:.
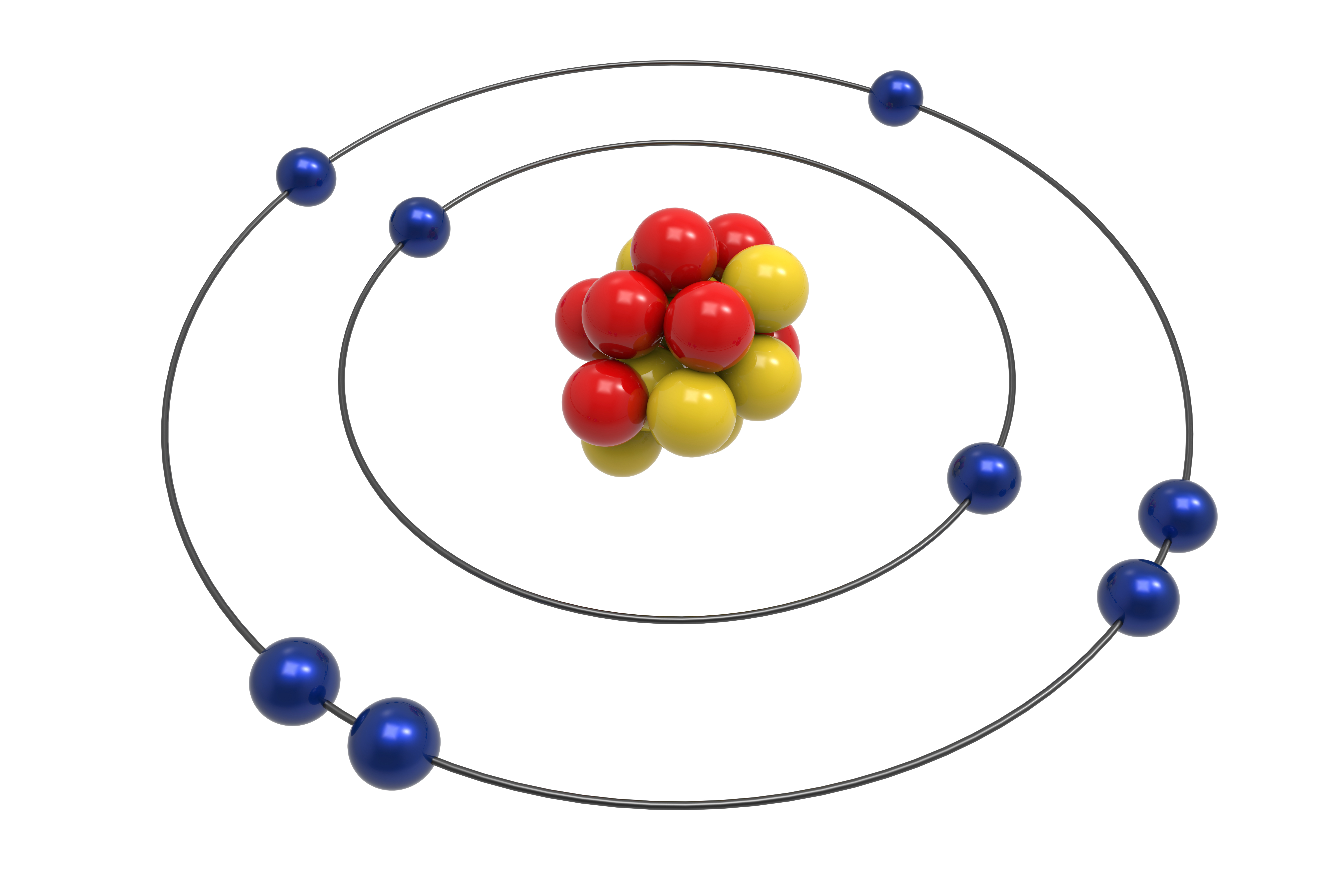
What Are The 4 Atomic Models

Rutherford Model Definition Facts Britannica

Describe The Model Of Atom Proposed By Rutherford Brainly In

A New Model Of The Atom Wikibooks Open Books For An Open World

The Diagram Below Shows Two Models Of The Atom Thompson S And Rutherford S Model Of Atoms The Models Brainly Com
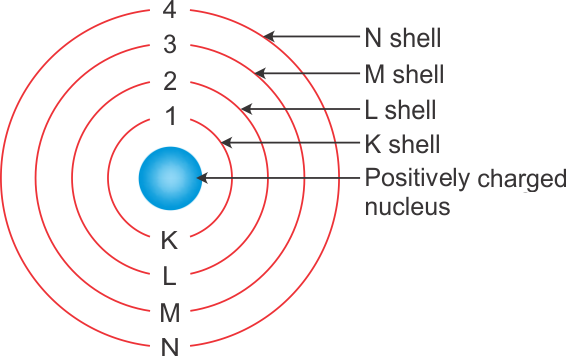
A Draw A Sketch Of Bohrs Model Of An Atom With Three Shells B If K L And M Shell Of An Atom Are Full Then What Would Be The Total Number

What Is An Atom It S A Question Of Physics The Atomic Age Linda Hall Library Kansas City Mo

Atom Model Universe Today

Quantum Model Of The Atom

Development Of The Nuclear Model Of The Atom Teaching Resources
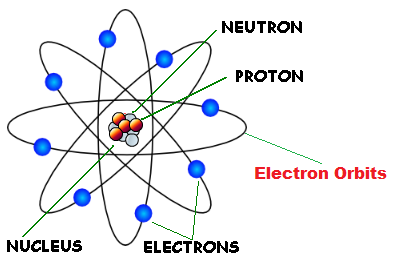
Discovery Of Atom And Nucleus Definition Examples Diagrams

Bohr Model Description Development Britannica
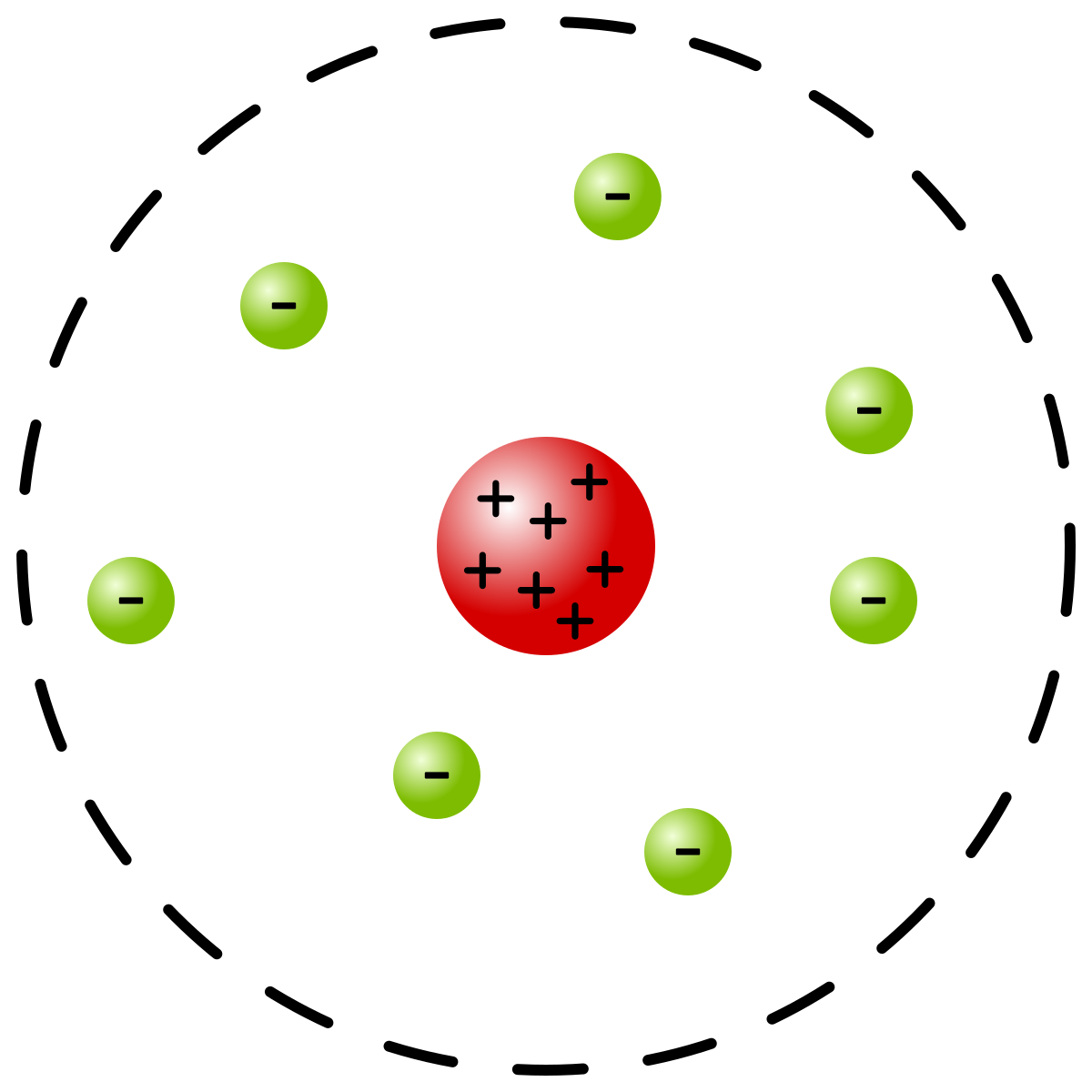
Rutherford Model Wikipedia
Bohr Atom The Planetary Model Of The Atom Objectives Ppt Download
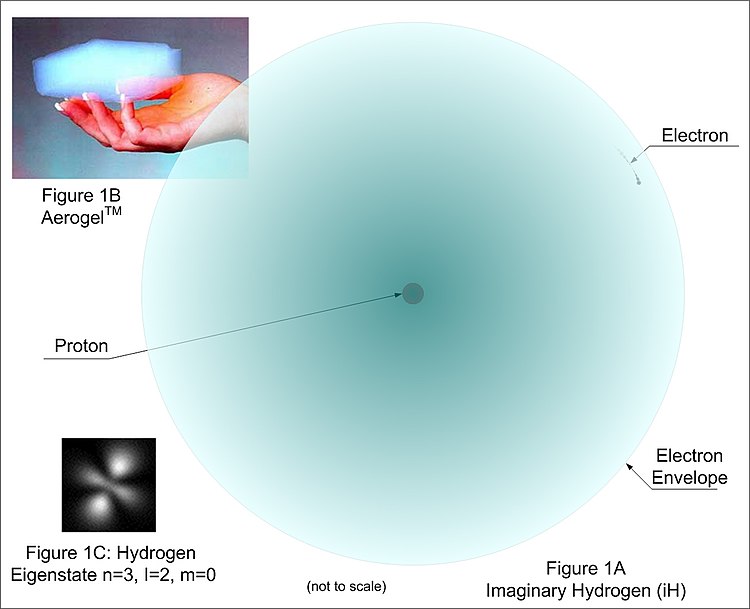
A New Model Of The Atom Wikibooks Open Books For An Open World
Bohr Model Of The Hydrogen Atom Equation Formula Limitations

A Timeline Of Atomic Models Did You Know That The Atomic Model Has By Intlink Education Medium
Difference Between Bohr And Rutherford S Atomic Models With Comparison Chart Bio Differences

Nuclear Model And Stability Physics Queensland Syllabus Openstax Cnx
Bohr Model Of The Atom Overview And Examples
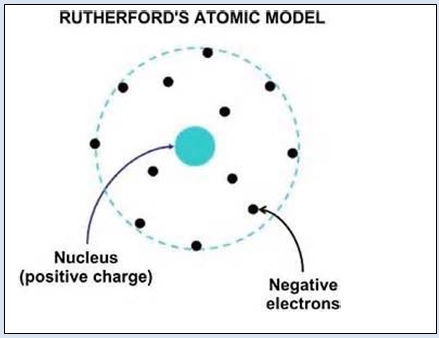
Rutherford S Model Of Atom Www Entelki In

Atom Models Of Atomic Structure Douglas Adomatis S Collection Of Atomic Structure Ideas

Students Made A Foldable Reviewing All The Dudes They Need To Know That Influenced The Development Of Th Chemistry Classroom Chemistry Class Teaching Chemistry
Q Tbn 3aand9gcsxptr Sahq633bw0mf3lxqhqutmsai3s1tldlvnomvv9nsuczc Usqp Cau
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Basic Model Of The Atom Atomic Theory
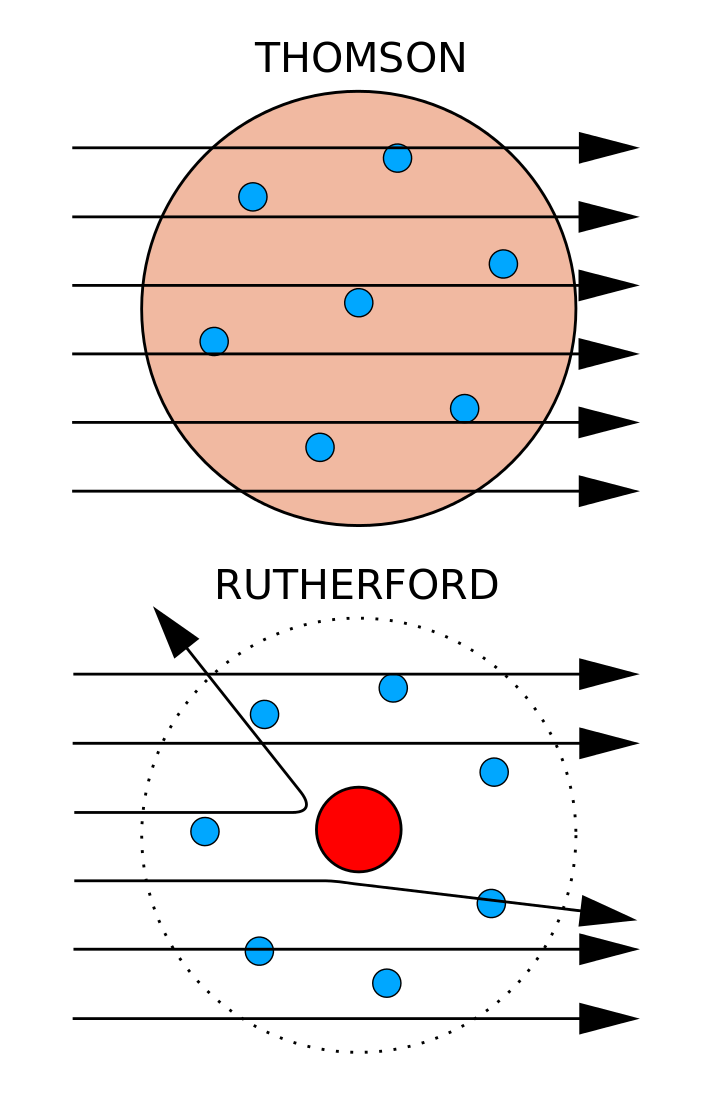
Experimental Evidence For The Structure Of The Atom

How Would You Describe The Structure Of An Atom A Plus Topper

The History Of The Atom Theories And Models Compound Interest
Q Tbn 3aand9gctufr0ni64i3v Vdr7daeu Abb4bun7cqlyrl6oxwxfiyj0hevp Usqp Cau

The Development Of The Atomic Model Wired

Bohr S Model Of An Atom With Postulates And Limitations Byju S
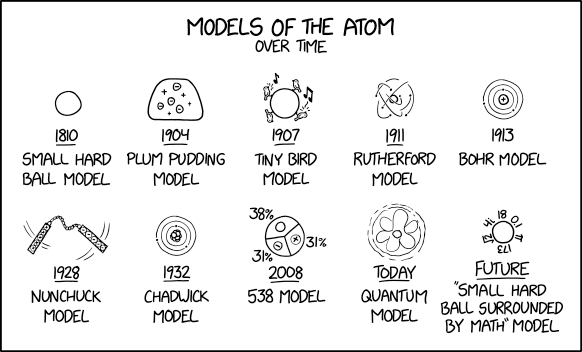
2100 Models Of The Atom Explain Xkcd

Atomic Models Learn Chemistry Class 9 Amrita Vidyalayam Elearning Network

2 Atomic Models School Of Materials Science And Engineering
How Bohr S Famous Model Of The Atom Was Created Kim Rendfeld

Rutherford Model Of The Atom

Dublin Schools Lesson Bohr S Model Of The Atom Whose Atomic Model First Accounted For Defined Energy Levels

Early Models Of The Atom Models Of Matter A Model Is A Tentative Description Of A System Or Theory That Accounts For All Of Its Known Properties Models Ppt Download

Explain The Thomson S Model Of An Atom With A Neat Diagram Brainly In
Bohr S Model Of Hydrogen Article Khan Academy
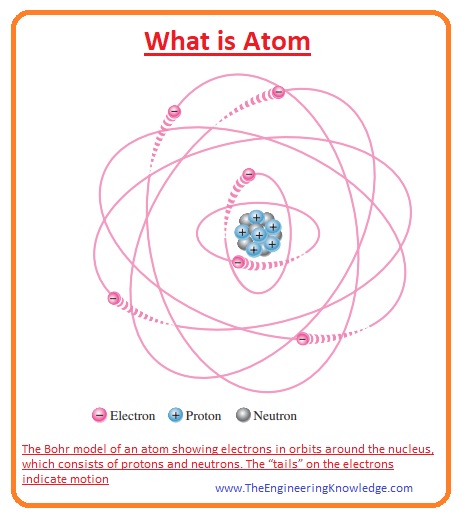
What Is Atom The Engineering Knowledge

Development Of Atomic Theory

Rutherford S Model Cbse 9 Science Chapter 4 Structure Of An Atom

The New Particle Like Model Of The Atom Authorstream
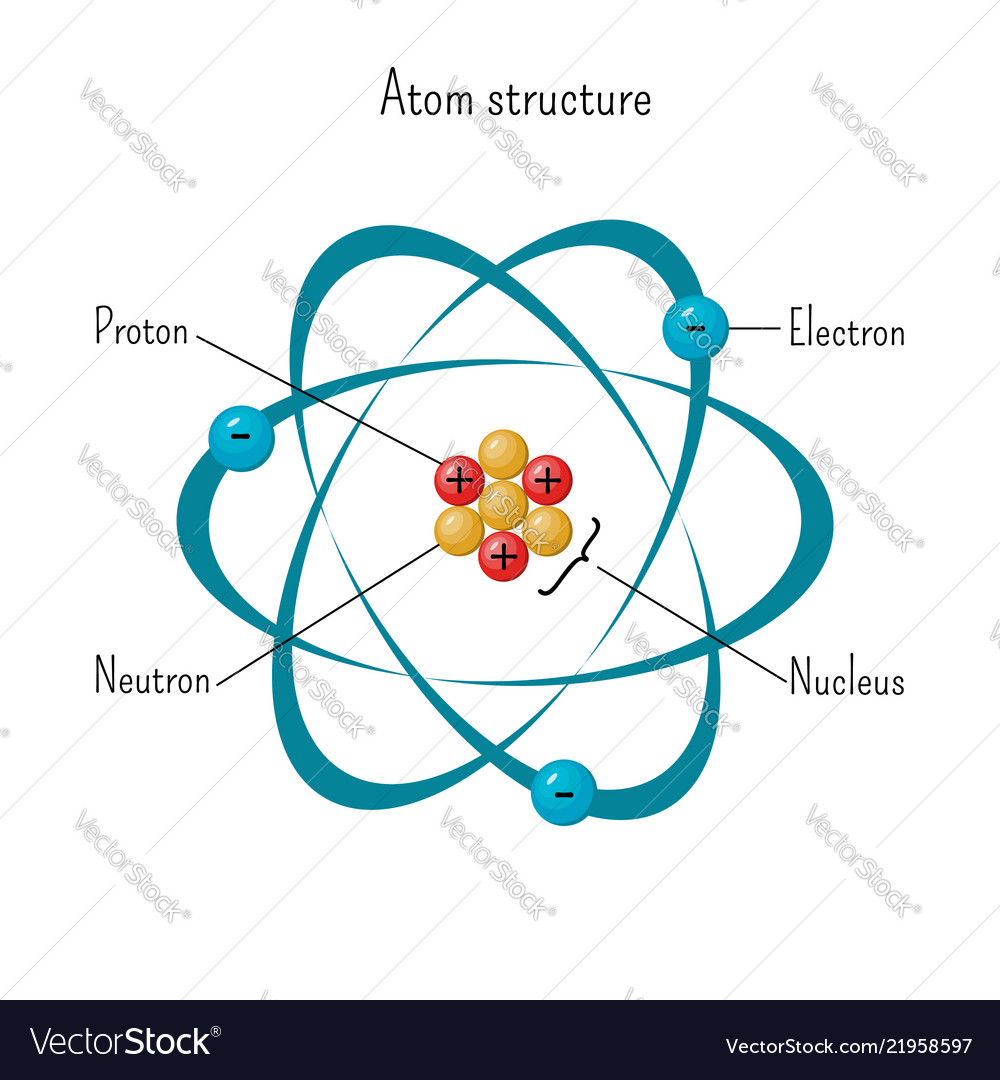
Simple Model Of Atom Structure With Electrons Vector Image

Thomson Atomic Model Description Image Britannica
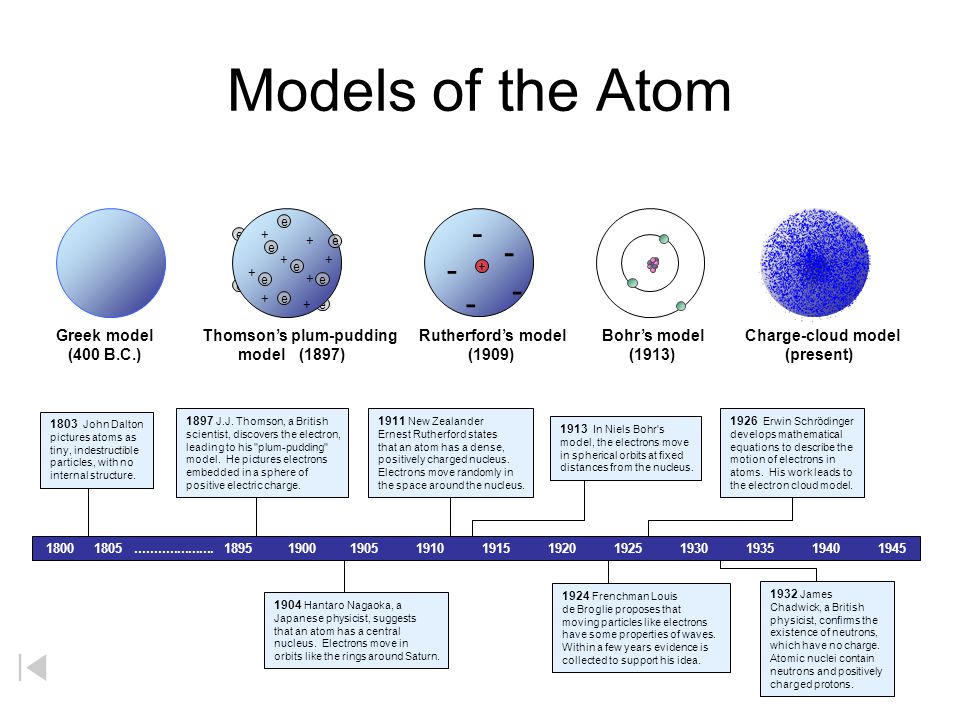
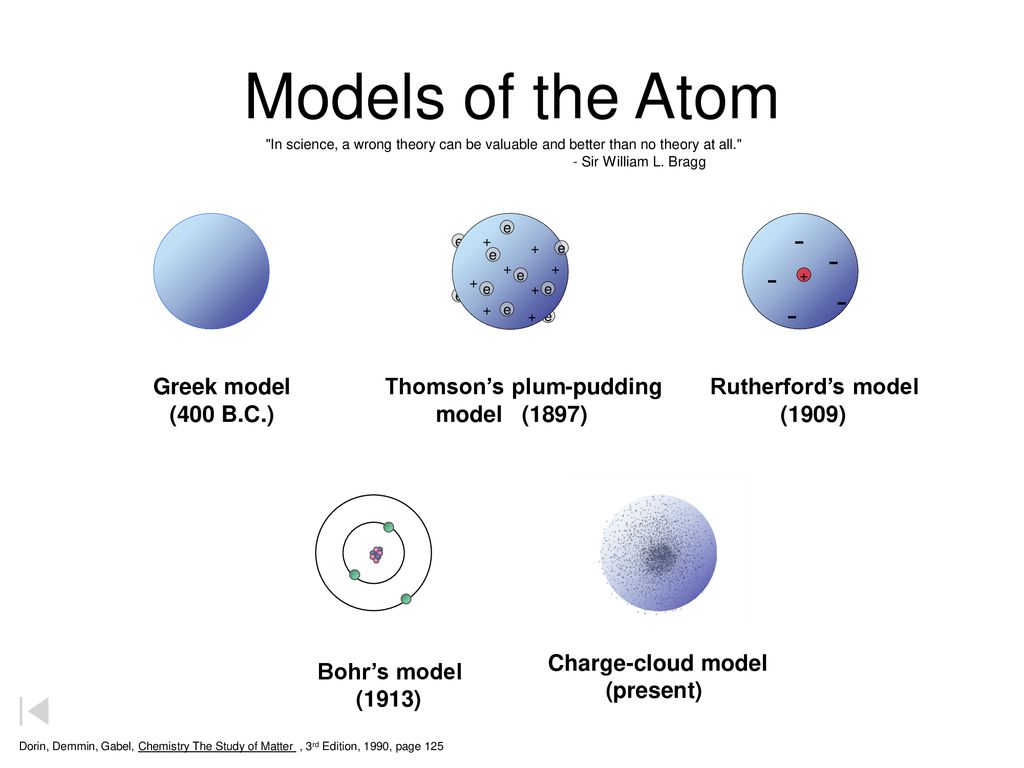
Models Of The Atom Dalton S Model 1803 Greek Model 400 B C Ppt Download

4 Describe Bohr S Model Of The Atom Scholr
Model Of Atom
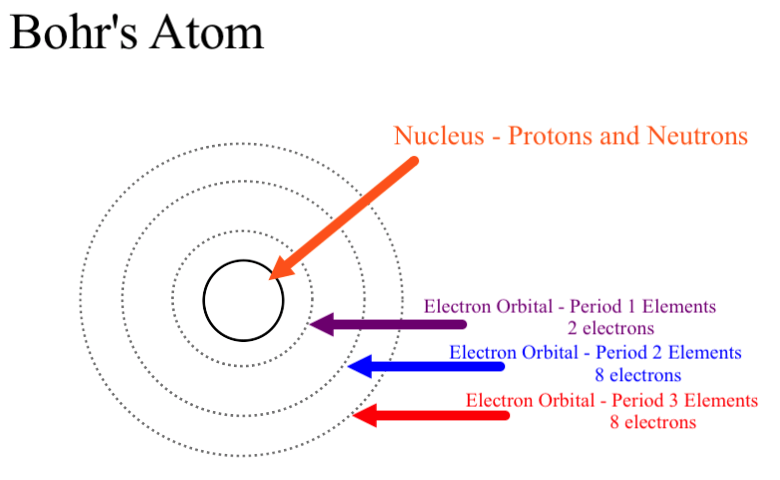
Why Could Bohr S Model Be Called A Planetary Model Of The Atom Socratic
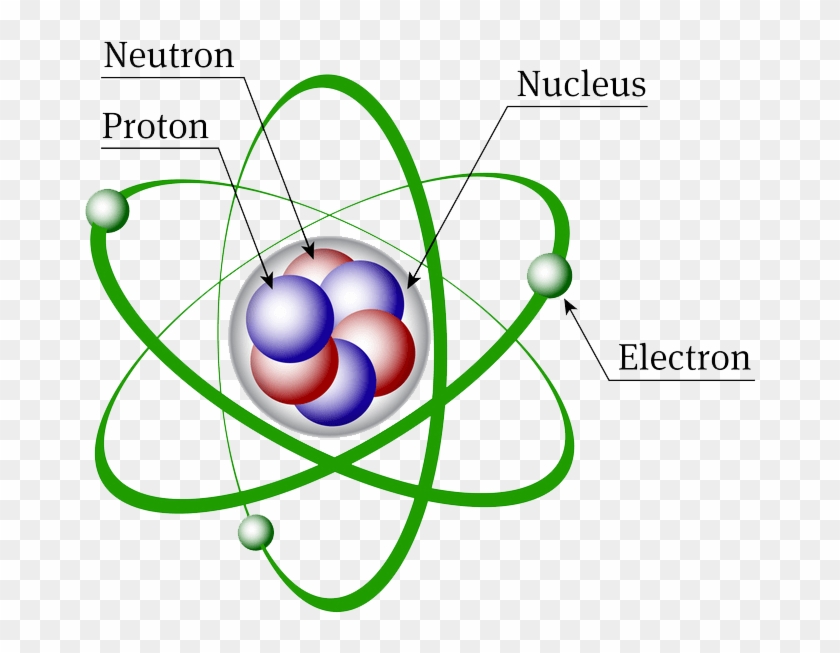
Atomic Structure Discovery Of Subatomic Particles Definition Nuclear Model Of An Atom Free Transparent Png Clipart Images Download

3 Atomic Models Villa

The Bohr Model Introduction To Chemistry
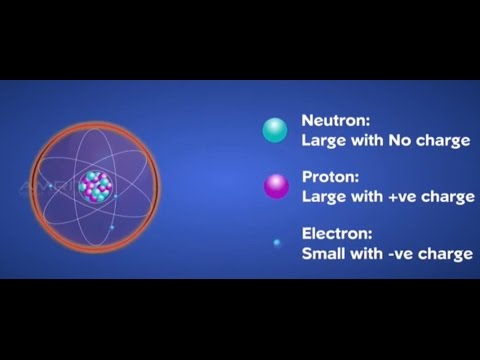
Thomson S Model Of An Atom Class 9 Tutorial Youtube
Q Tbn 3aand9gctxzw0nl5qfo6xdxbapjevah Daoikd7e2xpd9z 2zaskc3xib9 Usqp Cau

Bohr S Model Cbse Class 9 Science Chapter 4 Structure Of Atom
Q Tbn 3aand9gcthskfbn4p3dhcvbvvuxqnvbr3nxvfv57itvt2 Umniw6ok5vzp Usqp Cau
Www Sisd Net Cms Lib Tx Centricity Domain 1297 The History Of The Atom Notes Condensed Pdf

Neil Bohrs Atomic Model Learn About The Basics Of Electricity Bright Hub Engineering
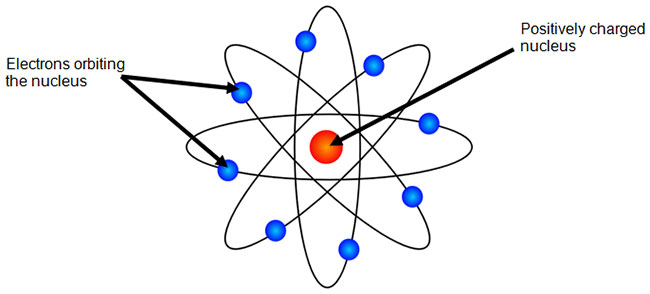
The Bohr Model Texas Gateway
Bohr Model Of The Atom Overview And Examples

5 Atom Models Atom Models Bohr Chemistry En Nuclear Plum Pudding Science Solid Sphere Wave Mechanical Glogster Edu Interactive Multimedia Posters
Developments Leading To The Bohr S Model Of Atom On Vimeo

The Development Of The Atomic Model Wired

Bohr Atomic Model

Bohr Model Of Atom Bohr S Postulates
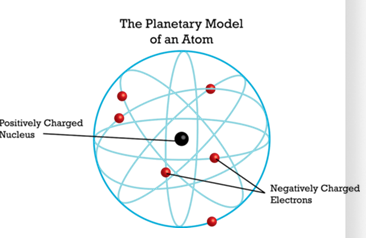
Rutherford S Planetary Model 1911 Rutherford S Sutori

Quantum Model Of The Atom

Atomic Structure Frcr Physics Notes
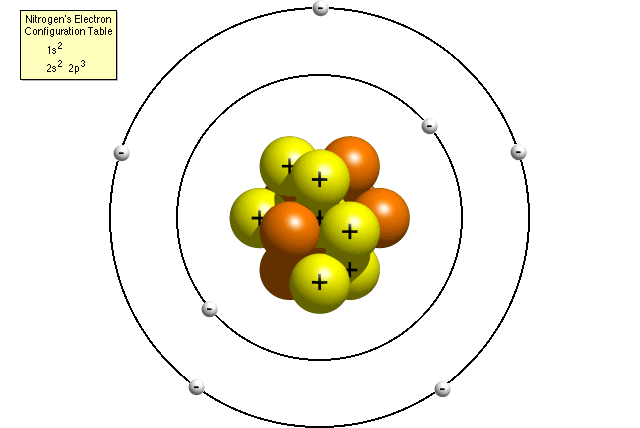
Questions And Answers How Do I Make A Model Of An Atom
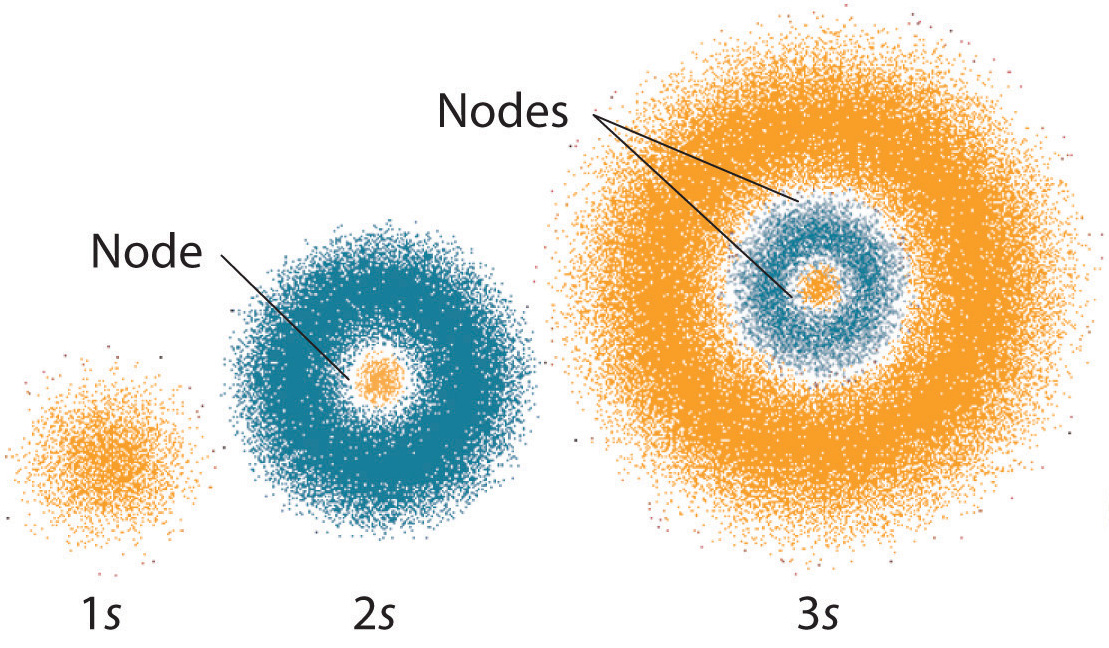
The Quantum Mechanical Model Of The Atom Article Khan Academy

5 Atom Models Atom Models Bohr Chemistry En Nuclear Plum Pudding Science Solid Sphere Wave Mechanical Glogster Edu Interactive Multimedia Posters
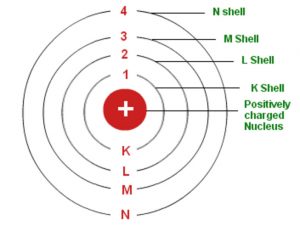
Bohr S Model Of An Atom Chemistry Class 11 Structure Of Atom

Bohr Model Of Atom Bohr S Postulates
What Is The Currently Accepted Model Of Atomic Structure Is There A More Recent Model Than The Bohr Or Bohr Sommerfeld That Has Been Observed By Physicists Quora

Rutherford Model Of Atom Class 11 Chemistry
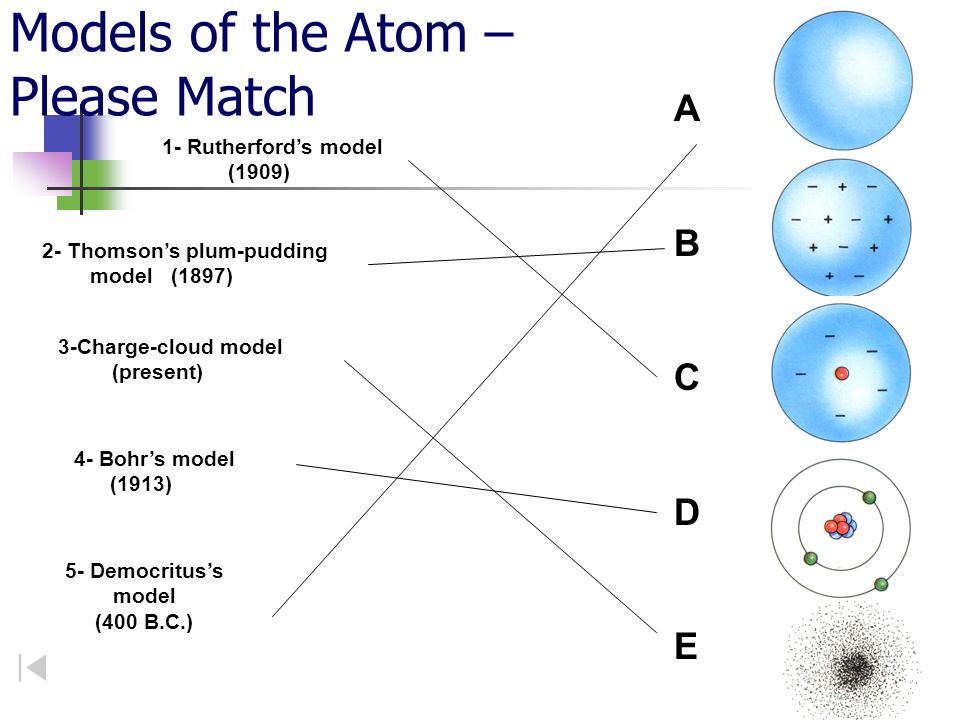
Models Of The Atom Please Match Ppt Video Online Download

Morgan S Chemistry Blog Atomic Models

Models Of The Atom The Atom Siyavula
Probing Difficulties With Quantum Atomic Models News Rsc Education

Thomson Model Of The Atom Plum Pudding Model

Rutherford S Atomic Model Chemistrygod

Models Of The Atom The Atom Siyavula
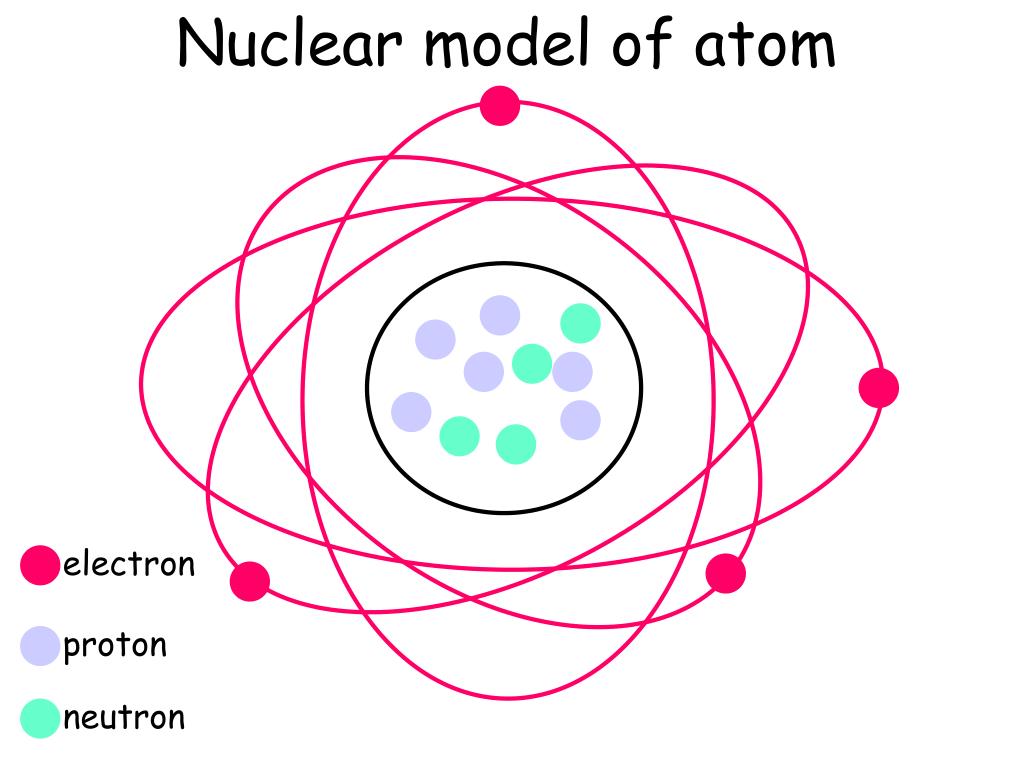
Ppt Nuclear Model Of Atom Powerpoint Presentation Free Download Id
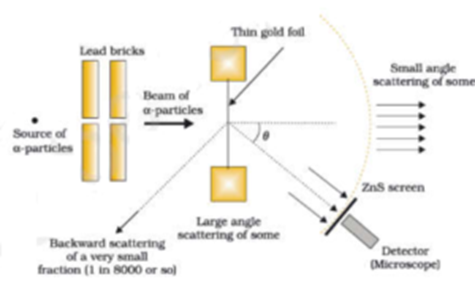
Rutherford S Model Of Atom Its Propositions Merits And Demerits

Image Result For Chlorine Atom Model 3d Project Atom Model Atom Model Project Atom Project

Ks4 Structure And History Of The Atom Full Lesson Teaching Resources
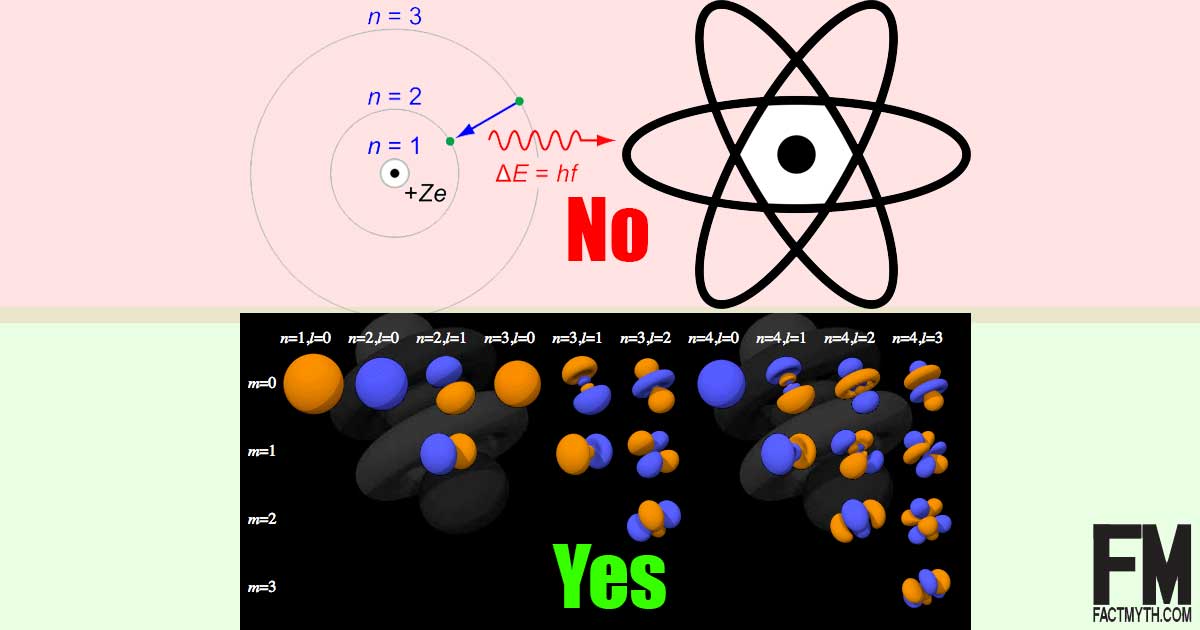
The Bohr Model Is The Most Accurate Model Of An Atom Fact Or Myth
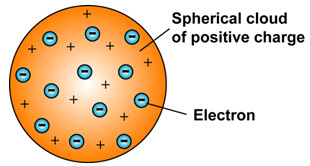
J J Thomson Model Of An Atom Class 9 Structure Of An Atom

Evolution Of The Model Of The Atom By Taylor R
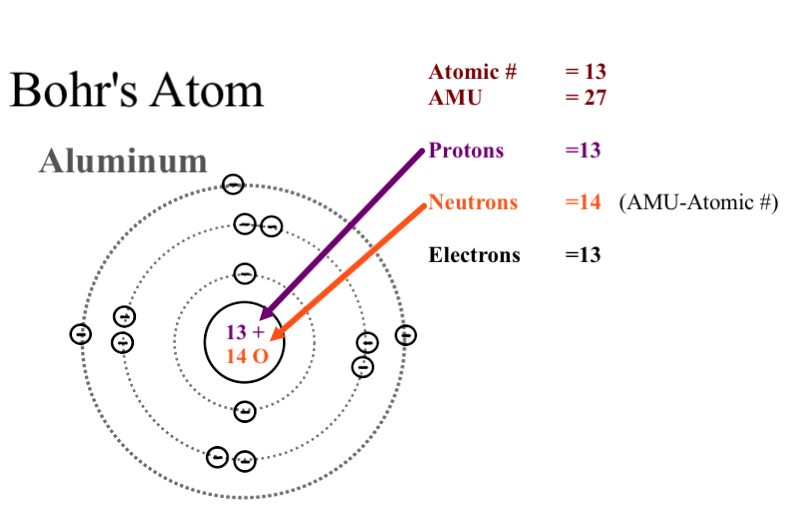
Why Could Bohr S Model Be Called A Planetary Model Of The Atom Socratic
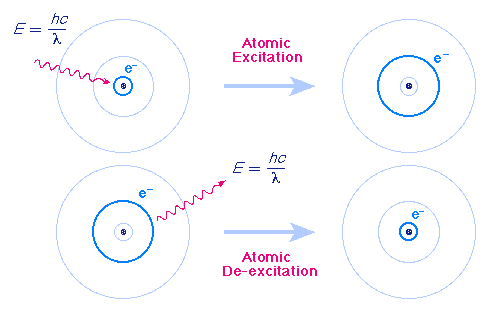
The Bohr Model
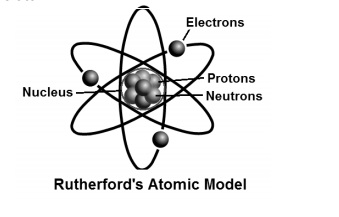
What Is Ruther Ford Model Of An Atom Imzm9ygg Chemistry Topperlearning Com
File Schrodinger Model Of The Atom Svg Wikimedia Commons

Models Of The Atom The Atom Siyavula

Learn Thomson S Model Of Atom In 2 Minutes
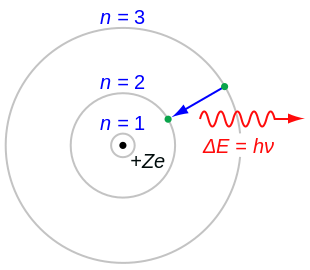
Bohr Model Wikipedia

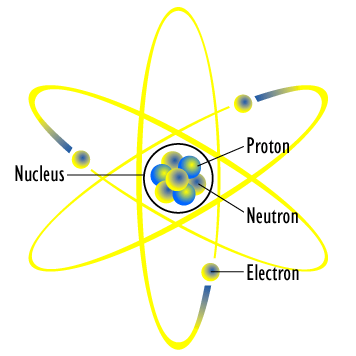
What Are The Parts Of An Atom



